Laboratory of Virology
Head:
Sandra Huerfano Meneses, Ph.D.
Where to Find Us:
BIOCEV, Průmyslová 595, Vestec u Prahy, 252 42
the first floor, Wing 3 (rooms 007, 008, 009, 011, 012, 013, 015)
Team MembersMethodology and Technical SupportScientific CollaborationsPublications |
TeachingProposed Topics of Theses for New StudentsStudent Theses in ProgressPast Student Theses |
Research Interests
 The Laboratory of Virology was established by Jitka Forstova at the Faculty of Sciences of Charles University in 1993. Until 2015, the virology laboratories functioned in the building Viničná 5, Prague 2 - Nové Město. At the end of 2015, the group relocated to BIOCEV complex in Vestec, the European Centre of Excellence in Biomedicine and Biotechnology that brought together 56 research teams from the Academy of Sciences of the Czech Republic and Charles University.
The Laboratory of Virology was established by Jitka Forstova at the Faculty of Sciences of Charles University in 1993. Until 2015, the virology laboratories functioned in the building Viničná 5, Prague 2 - Nové Město. At the end of 2015, the group relocated to BIOCEV complex in Vestec, the European Centre of Excellence in Biomedicine and Biotechnology that brought together 56 research teams from the Academy of Sciences of the Czech Republic and Charles University.
Our research group studies the biology of small, non-enveloped DNA viruses. It offers a unique environment for both basic and applied research, with many opportunities for undergraduate and graduate students.
Our main focus is on mouse polyomavirus (MPyV) as a model virus for human polyomaviruses (BKPyV, JCPyV and MCPyV), which have the potential to cause severe illnesses in immunocompromised patients. For example, MCPyV causes aggressive Merkel cell skin carcinoma, while BKPyV leads to nephropathy and is the most common cause of kidney transplant failure. Since 2022, studies on the human BK virus have been carried out in our Laboratory.
Currently, our research covers three major topics:
1) Polyomavirus trafficking to the nucleus and interactions of the viral proteins with host cell partners
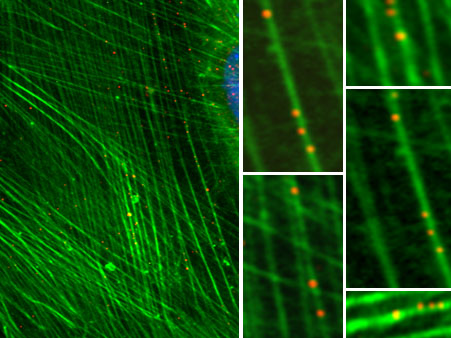
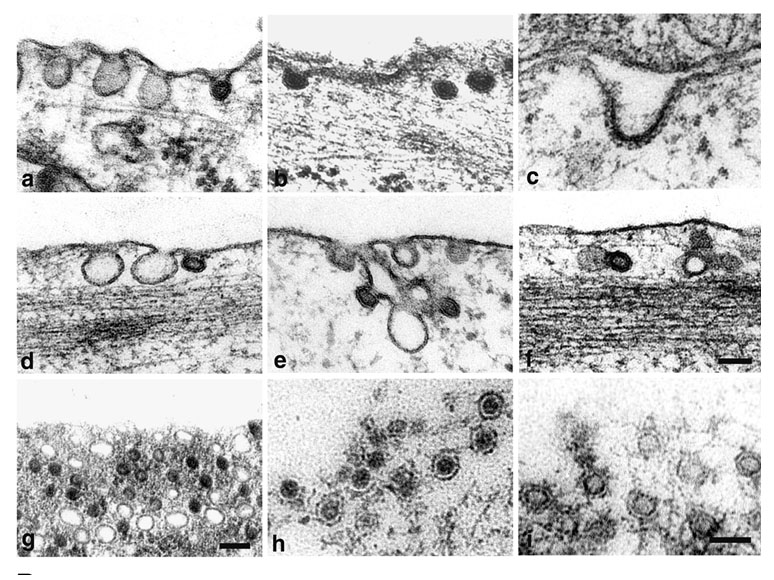 We have studied the role of endosomal cellular structures and the cytoskeleton in productive MPyV infection. We have revealed that the productive endocytic pathway begins with endocytosis of the virus in monopinocytic vesicles derived from membrane rafts, followed by the consecutive transport of the virus from the early endosomes to late acidic compartments, from which it is sorted to the endoplasmic reticulum (ER). An acidic endosome pH and dynein-mediated vesicle movement are required for the infectious pathway. Virions are released from the ER to the cytosol with the help of minor structural proteins. In the cytosol, partially disassembled virions interact with importins for translocation to the nucleus.
We have studied the role of endosomal cellular structures and the cytoskeleton in productive MPyV infection. We have revealed that the productive endocytic pathway begins with endocytosis of the virus in monopinocytic vesicles derived from membrane rafts, followed by the consecutive transport of the virus from the early endosomes to late acidic compartments, from which it is sorted to the endoplasmic reticulum (ER). An acidic endosome pH and dynein-mediated vesicle movement are required for the infectious pathway. Virions are released from the ER to the cytosol with the help of minor structural proteins. In the cytosol, partially disassembled virions interact with importins for translocation to the nucleus.
We have also studied the properties, interactions and functions of MPyV early tumour antigens and late capsid proteins for years, often in collaboration with foreign laboratories. Among other findings, we revealed that the oncogenic protein MT is a truly transmembrane protein located in clumps in membrane raft structures, an arrangement important for MT’s ability to transform cells. Furthermore, we identified new cellular interacting partners of the major capsid protein, VP1, namely the pleiotropic cell regulator YY1, apparently involved in viral minichromosome remodelling connected with virion morphogenesis, as well as heat shock protein 90 and microtubules. The direct binding of VP1 pentamers to microtubules affects cell cycle progression. Moreover, the viroporin-like properties of the minor structural proteins VP2 and VP3 have been revealed; their hydrophobic domains interact with endocellular membranes and are important for virion exit from the ER.
2) The host cellular immune response to polyomaviruses and viral components
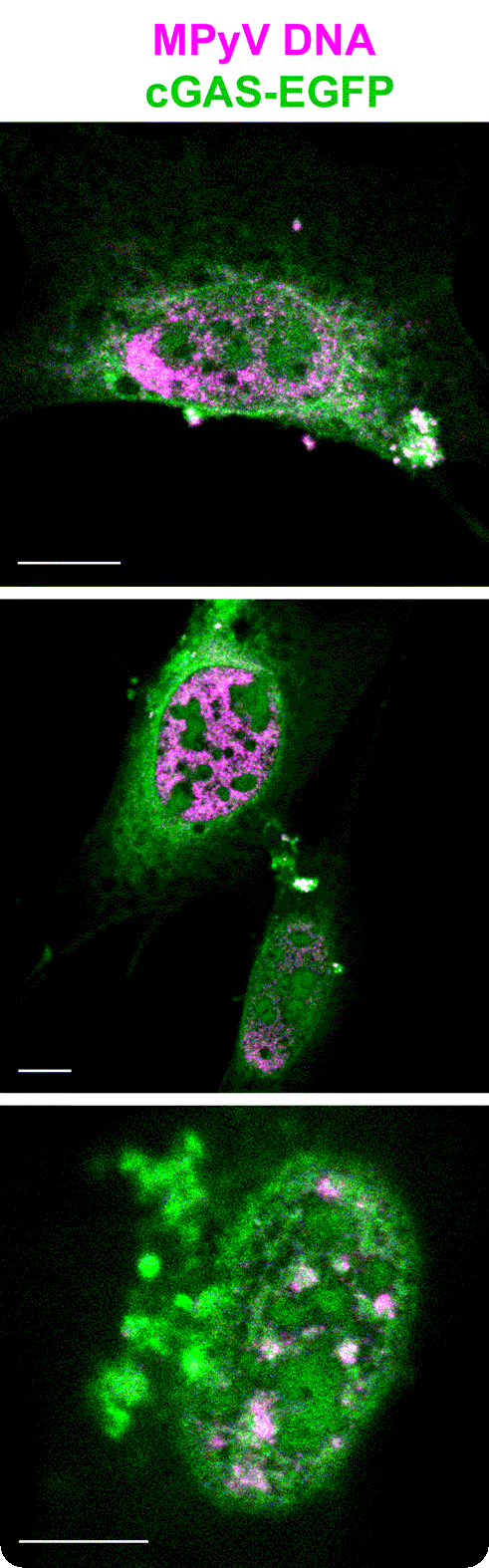 A significant portion of our scientific research has been directed towards the cellular immune responses to polyomavirus infections and polyomavirus-based chimeric VLPs. In recent years, we have focused on the mechanisms of the innate immune sensing of polyomavirus DNA. Among other findings, we revealed that viral DNA sensing in polyomavirus-infected cells is mediated by p204 (a mouse analogue of human IFI16) and cGAS DNA sensors. Further, our studies contributed to uncovering the cellular sensing of polyomavirus infection via Toll-like receptor 4 (TLR4). TLR4 activation leads to the production of interleukin 6 and other cytokines that shape the cellular microenvironment to promote the development of a cancer-associated fibroblast-like phenotype.
A significant portion of our scientific research has been directed towards the cellular immune responses to polyomavirus infections and polyomavirus-based chimeric VLPs. In recent years, we have focused on the mechanisms of the innate immune sensing of polyomavirus DNA. Among other findings, we revealed that viral DNA sensing in polyomavirus-infected cells is mediated by p204 (a mouse analogue of human IFI16) and cGAS DNA sensors. Further, our studies contributed to uncovering the cellular sensing of polyomavirus infection via Toll-like receptor 4 (TLR4). TLR4 activation leads to the production of interleukin 6 and other cytokines that shape the cellular microenvironment to promote the development of a cancer-associated fibroblast-like phenotype.
Studies of immune responses to VLP nanostructures that can be used therapeutically are of interest. First, mice inoculated intranasally with EGFP chimeric VLPs were found to strongly produce antibodies against the VP1 capsid protein but not EGFP itself. Second, studies on dendritic cells revealed that VLPs induced interleukin 12 secretion but not the upregulation of co-stimulatory molecules or maturation markers. These results suggest that polyomavirus-derived VLPs are promising delivery and adjuvant vehicles but need further development and improvement of the antigen presentation and immunization strategies.
3) The use of mouse polyomavirus-like particles (MPyV-VLPs) in nanotechnology
 In the context of nanotechnology, VLPs have several unique biochemical properties (biodegradability and, unlike other polymers, a well-defined shape and structure) that facilitate their use as scaffolds for the construction of nano-objects for biomedical applications. We modified VP1 within VLPs to change the natural tropism of the particles for specific cancer cell receptors. As an experimental cancer cell model, we used androgen-resistant human prostate tumour cells expressing a distinct surface cancer marker, prostate membrane antigen (PSMA). Furthermore, we previously prepared species-specific antigens based on recombinant VP1 that were used only for serological studies. In cooperation with the VIDIA company, we have developed a multiplex diagnostic confirmatory line immunoassay based on recombinant VLP technology to detect subtype-specific antibodies against BKPyV, MCPyV and JCPyV for clinical use. In cooperation with the Dyntec company, we have developed veterinary vaccines against porcine circovirus 2 based on chimeric polyomavirus structures.
In the context of nanotechnology, VLPs have several unique biochemical properties (biodegradability and, unlike other polymers, a well-defined shape and structure) that facilitate their use as scaffolds for the construction of nano-objects for biomedical applications. We modified VP1 within VLPs to change the natural tropism of the particles for specific cancer cell receptors. As an experimental cancer cell model, we used androgen-resistant human prostate tumour cells expressing a distinct surface cancer marker, prostate membrane antigen (PSMA). Furthermore, we previously prepared species-specific antigens based on recombinant VP1 that were used only for serological studies. In cooperation with the VIDIA company, we have developed a multiplex diagnostic confirmatory line immunoassay based on recombinant VLP technology to detect subtype-specific antibodies against BKPyV, MCPyV and JCPyV for clinical use. In cooperation with the Dyntec company, we have developed veterinary vaccines against porcine circovirus 2 based on chimeric polyomavirus structures.
Members of the Laboratory are also part of the research team "Interactions of Viral and Cellular Structures during Viral Infection and Development of Nanostructures for Medical and Veterinary Purposes" operating within the programme "Cell Biology and Virology" at the Biotechnology and Biomedicine Center of the Czech Academy of Sciences and Charles University BIOCEV. Additionally, they are involved in research programs within the National Institute of Virology and Bacteriology, which is a platform bringing together leading virological and microbiological research institutions in the Czech Republic.





















