Laboratory of Immunotherapy
Head:
RNDr. Michal Šmahel, Ph.D.
Where to Find Us:
BIOCEV, Průmyslová 595, Vestec, 252 50
the first floor, Wing 3 (rooms 004, 007, 015, 017)
Team MembersMethodology and Technical SupportScientific CollaborationsPublications |
TeachingProposed Topics of Theses for New StudentsStudent Theses in ProgressPast Student Theses |
Research Interests
The Laboratory of Molecular and Tumor Virology was established in 2015 by a group of researchers who transferred from the Institute of Hematology and Blood Transfusion (IHBT) in Prague. They joined the research team "Immunization Against Tumors Induced by Human Viruses" within the Cellular Biology and Virology programme at the then newly established BIOCEV research center in Vestec, near Prague. The Laboratory’s activities focus on the immunotherapy of virus-induced tumors, which is experimentally tested on mouse tumor models involving human papillomaviruses.
 In the immunotherapy of tumors, anti-tumor DNA vaccines administered biolistically with a gene gun are used. This immunization is mainly directed against the E7 oncoprotein of HPV-16. To enhance the immunity of CD8+ T cells, a fusion gene carrying, in addition to the E7 oncogene, mutated to reduce its oncogenic potential, a universal helper epitope PADRE, designed in silico to activate CD4+ T cells, was created. We seek to further enhance DNA vaccination-activated immunity by stimulating cells of innate immunity. For this purpose, we have found immunostimulatory CpG motifs in the form of the synthetic oligonucleotide ODN1826 to be successful. In collaboration with the Institute of Organic Chemistry and Biochemistry, we also test agonists of the STING protein
In the immunotherapy of tumors, anti-tumor DNA vaccines administered biolistically with a gene gun are used. This immunization is mainly directed against the E7 oncoprotein of HPV-16. To enhance the immunity of CD8+ T cells, a fusion gene carrying, in addition to the E7 oncogene, mutated to reduce its oncogenic potential, a universal helper epitope PADRE, designed in silico to activate CD4+ T cells, was created. We seek to further enhance DNA vaccination-activated immunity by stimulating cells of innate immunity. For this purpose, we have found immunostimulatory CpG motifs in the form of the synthetic oligonucleotide ODN1826 to be successful. In collaboration with the Institute of Organic Chemistry and Biochemistry, we also test agonists of the STING protein
Activation of immune responses alone usually has only a limited anti-tumor effect because various mechanisms that suppress the anti-tumor response are induced in the tumor microenvironment. A common mechanism is the activation of immune checkpoints, such as PD-1, CTLA-4, or TIM-3 proteins. Therefore, our immunotherapy also involves monoclonal antibodies that block these molecules, an increasingly successful approach in clinical practice. However, its efficacy is limited to a minority of patients in most tumor types. This may be due to other mechanisms by which tumor cells escape the host immune system
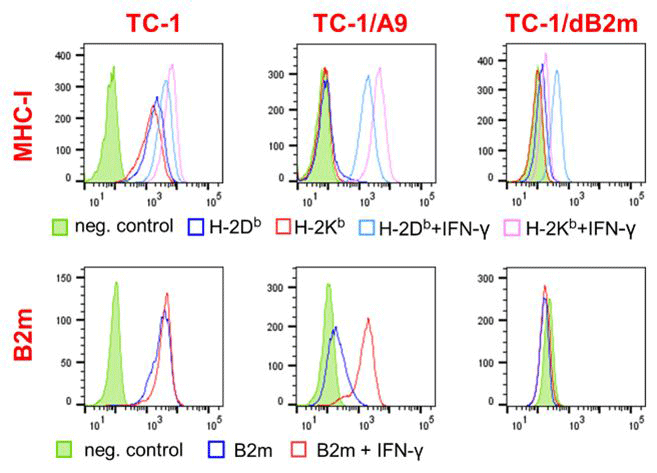 The main antitumor effect of antibodies blocking PD-1, CTLA-4, or TIM-3 receptors is to increase the activity of cytotoxic T lymphocytes that recognize tumor cells presenting epitopes bound to major histocompatibility complex class 1 (MHC-I) molecules on their surface. However, tumors often manage to evade the intervention of these immune cells due to the genetic instability of tumor cells and the selection of variants with reduced expression of MHC-I molecules, one of the most widespread escape mechanisms in human tumors. Therefore, in developing a mouse model, we focused on deriving oncogenic cell clones with reduced MHC-I molecules expressed on the cell surface. This reduction can be either reversible or irreversible. In the case of reversible change, which can be caused by epigenetic regulation, the surface expression of MHC-I molecules can be restored by induction with, e.g., interferon γ (IFN-γ). Irreversible changes are caused by genetic mutations, which may have different phenotypic manifestations. For example, complete loss of surface expression of MHC-I occurs when β2-microglobulin production is impaired. We have previously obtained tumor clones with reversibly reduced expression of MHC-I molecules from mouse tumors, where these clones were naturally selected after DNA immunization against the E7 oncoprotein. We have now prepared clones in which the MHC-I light chain gene (encoding β2-microglobulin) or the heavy chain genes have been inactivated using the CRISPR-Cas9 system, thus achieving irreversible loss of MHC-I surface expression. These clones will allow us to improve the efficacy of immunotherapy against partially resistant tumors and to study the effects of β2-microglobulin, which may also act as a growth factor to influence tumor behavior and the success of therapy.
The main antitumor effect of antibodies blocking PD-1, CTLA-4, or TIM-3 receptors is to increase the activity of cytotoxic T lymphocytes that recognize tumor cells presenting epitopes bound to major histocompatibility complex class 1 (MHC-I) molecules on their surface. However, tumors often manage to evade the intervention of these immune cells due to the genetic instability of tumor cells and the selection of variants with reduced expression of MHC-I molecules, one of the most widespread escape mechanisms in human tumors. Therefore, in developing a mouse model, we focused on deriving oncogenic cell clones with reduced MHC-I molecules expressed on the cell surface. This reduction can be either reversible or irreversible. In the case of reversible change, which can be caused by epigenetic regulation, the surface expression of MHC-I molecules can be restored by induction with, e.g., interferon γ (IFN-γ). Irreversible changes are caused by genetic mutations, which may have different phenotypic manifestations. For example, complete loss of surface expression of MHC-I occurs when β2-microglobulin production is impaired. We have previously obtained tumor clones with reversibly reduced expression of MHC-I molecules from mouse tumors, where these clones were naturally selected after DNA immunization against the E7 oncoprotein. We have now prepared clones in which the MHC-I light chain gene (encoding β2-microglobulin) or the heavy chain genes have been inactivated using the CRISPR-Cas9 system, thus achieving irreversible loss of MHC-I surface expression. These clones will allow us to improve the efficacy of immunotherapy against partially resistant tumors and to study the effects of β2-microglobulin, which may also act as a growth factor to influence tumor behavior and the success of therapy.
 Although tumor cells with reduced expression of MHC-I molecules are insensitive to cytotoxic T cells, they are usually more sensitive to NK cell killing and can also be destroyed by other innate immune cells, e.g., macrophages. Therefore, we are interested in developing combined immunotherapeutic approaches that activate different cells of adaptive and innate immunity and are effective against tumor cells with different expression levels of MHC-I molecules. Thus, one of the goals of combination immunotherapy is to convert M2 pro-tumor macrophages into M1 anti-tumor macrophages, which could also effectively inhibit the growth of tumors induced by cells with reduced MHC-I molecules.
Although tumor cells with reduced expression of MHC-I molecules are insensitive to cytotoxic T cells, they are usually more sensitive to NK cell killing and can also be destroyed by other innate immune cells, e.g., macrophages. Therefore, we are interested in developing combined immunotherapeutic approaches that activate different cells of adaptive and innate immunity and are effective against tumor cells with different expression levels of MHC-I molecules. Thus, one of the goals of combination immunotherapy is to convert M2 pro-tumor macrophages into M1 anti-tumor macrophages, which could also effectively inhibit the growth of tumors induced by cells with reduced MHC-I molecules.
 The tumor microenvironment is a complex of various cell types that contribute to tumor development or, conversely, to antitumor responses by different mechanisms. One of our goals is to characterize these mechanisms and monitor their impact on immunotherapeutic approaches. Therefore, we isolate cells from tumors and characterize them by flow cytometry and single-cell RNA sequencing. We also focus on the spatial heterogeneity of tumors in terms of both tumor cells (in our model, we are particularly interested in the heterogeneity of MHC-I expression) and immune cells.
The tumor microenvironment is a complex of various cell types that contribute to tumor development or, conversely, to antitumor responses by different mechanisms. One of our goals is to characterize these mechanisms and monitor their impact on immunotherapeutic approaches. Therefore, we isolate cells from tumors and characterize them by flow cytometry and single-cell RNA sequencing. We also focus on the spatial heterogeneity of tumors in terms of both tumor cells (in our model, we are particularly interested in the heterogeneity of MHC-I expression) and immune cells.
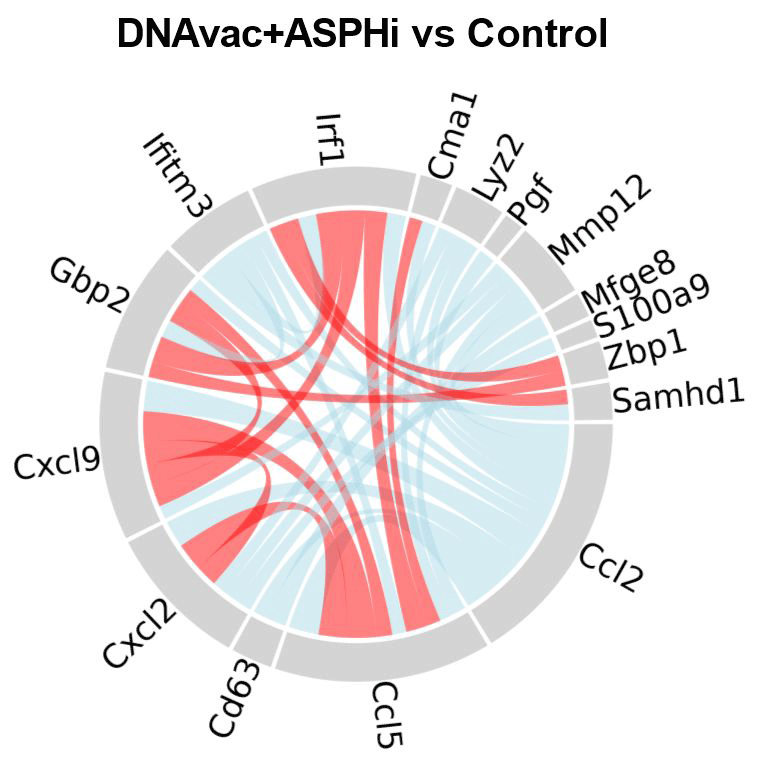
The laboratory is also interested in the enzyme aspartate β-hydroxylase (ASPH). We investigate its involvement in the signaling pathways of carcinogenesis in different types of tumors and use it as a therapeutic target by inhibiting its enzymatic activity with small molecule inhibitors. Since we have shown that ASPH inhibition enhances the adaptive immune response induced by DNA vaccination, we seek to incorporate ASPH inhibitors into combination immunotherapy of cancer and to elucidate the mechanisms by which ASPH inhibition contributes to the immune response.
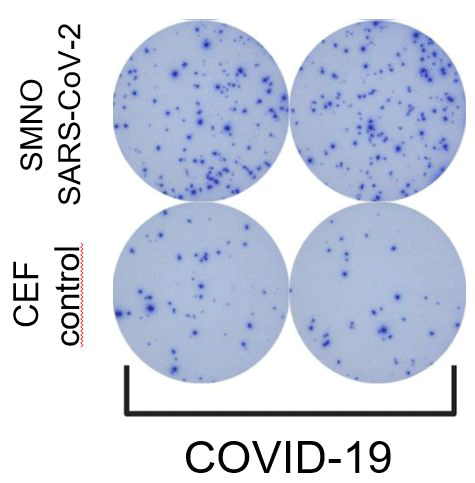 During the COVID-19 pandemic, we developed ELISPOT assays to detect SARS-CoV-2 specific memory T and B lymphocytes. We used these assays to analyze the immune response in patients with varying severity of COVID-19 disease.
During the COVID-19 pandemic, we developed ELISPOT assays to detect SARS-CoV-2 specific memory T and B lymphocytes. We used these assays to analyze the immune response in patients with varying severity of COVID-19 disease.





















