Laboratory of Biology of Yeast Colonies
Head:
prof. RNDr. Zdena Palková, CSc.
Department of Genetics and Microbiology
Study consultant and guarantor of M.Sc. study programme Genetics, Molecular Biology and Virology
BIOCEV Vestec, 2NP, room A2.076
Viničná 5, 2NP, room 116
Where to Find Us:
BIOCEV, Průmyslová 595, Vestec, 252 42
the first floor, Wing 6 (rooms 072, 074, 075, 076, 077, 078, 079)
Viničná 5, Prague 2 - New Town, 128 43
the ground floor (room P8), the first floor (rooms 116, 118A)
Team MembersMethodology and Technical SupportScientific CollaborationsPublications |
TeachingProposed Topics of Theses for New StudentsStudent Theses in ProgressPast Student Theses |
Research Interests
Further information can be also found at http://web.natur.cuni.cz/~zdenap/ website
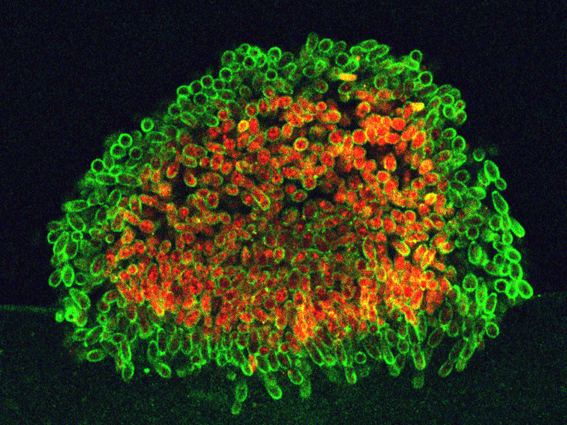 It has long been customary to categorize organisms into unicellular and multicellular groups. However, recent years have shown that the boundaries separating these two groups are not as strict as they might seem. Most unicellular organisms, including yeasts, can form organized multicellular structures such as biofilms and colonies under certain conditions. In natural environments, these structures represent the predominant form of microbial life, enabling better survival under adverse conditions.
It has long been customary to categorize organisms into unicellular and multicellular groups. However, recent years have shown that the boundaries separating these two groups are not as strict as they might seem. Most unicellular organisms, including yeasts, can form organized multicellular structures such as biofilms and colonies under certain conditions. In natural environments, these structures represent the predominant form of microbial life, enabling better survival under adverse conditions.
Yeasts that inhabit solid surfaces create multicellular structures - colonies - with distinct morphologies and organization. During the development of such a colony, individual yeast cells differentiate and form specifically localized subpopulations, each with specific roles within the structure. Thus, yeast colonies behave like primitive multicellular organisms: cells communicate with each other, synchronize their development, and differentiate into primitive "tissues." This differentiation often contributes to the prolonged viability of the entire cell population within the colony.
The Laboratory of Biology of Yeast Colonies has for a long time studied signaling, differentiation, adaptation, and survival in multicellular yeast populations - colonies and biofilms. Understanding the molecular and cellular mechanisms involved in the formation and development of microbial populations can aid in the fight against microbial biofilms, which pose significant challenges in medicine. Additionally, these insights can contribute to understanding the principles important for the formation and development of tissues in higher organisms, including humans.
Currently, the Laboratory focuses on two main research areas:
1) Development and differentiation of biofilms/colonies formed by "wild" strains of Saccharomyces cerevisiae
 Unlike the smooth colonies formed by strains of S. cerevisiae cultivated for a long time under laboratory conditions, "wild" strains of S. cerevisiae isolated from nature typically produce colonies with highly structured morphology, in which cells are connected by an extracellular matrix that forms channels and spaces for nutrient and waste flow. These colonies closely resemble natural biofilms and are characterized by specific internal organization. During colony development, cells differentiate into subpopulations with distinct localizations and defense mechanisms, providing the colony with high resistance to various environmental stressors. Cellular subpopulations with active drug-resistance transport proteins (multidrug resistance), as well as those producing extracellular matrix, form in specific locations within the colony. When "wild" S. cerevisiae strains are cultivated on glucose-rich media, they can undergo "domestication," reorganizing their colonial lifestyle and deactivating some defense mechanisms. This "domestication" is accompanied by changes in gene expression leading to altered metabolism and the cessation of extracellular matrix production; however, it is a reversible process.
Unlike the smooth colonies formed by strains of S. cerevisiae cultivated for a long time under laboratory conditions, "wild" strains of S. cerevisiae isolated from nature typically produce colonies with highly structured morphology, in which cells are connected by an extracellular matrix that forms channels and spaces for nutrient and waste flow. These colonies closely resemble natural biofilms and are characterized by specific internal organization. During colony development, cells differentiate into subpopulations with distinct localizations and defense mechanisms, providing the colony with high resistance to various environmental stressors. Cellular subpopulations with active drug-resistance transport proteins (multidrug resistance), as well as those producing extracellular matrix, form in specific locations within the colony. When "wild" S. cerevisiae strains are cultivated on glucose-rich media, they can undergo "domestication," reorganizing their colonial lifestyle and deactivating some defense mechanisms. This "domestication" is accompanied by changes in gene expression leading to altered metabolism and the cessation of extracellular matrix production; however, it is a reversible process.
The goal of the Laboratory's research in this area is to identify new regulatory mechanisms contributing to biofilm colony formation and cell differentiation. The focus is particularly on mechanisms involved in resistance to environmental stressors and those involved in the "domestication" of the respective yeast strains.
2) Development and differentiation of smooth colonies formed by laboratory and "domesticated" strains of Saccharomyces cerevisiae
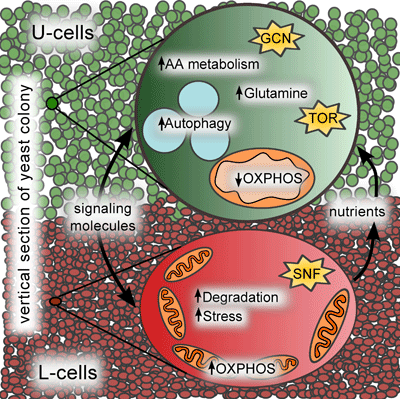 One of the typical features of multicellular organisms is their ability to produce and receive signals over long distances. Yeast colonies utilize a simple volatile compound, ammonia, for signaling within the colony, which is produced in pulses. This results in the synchronization of development between neighboring colonies, and in Candida mogii yeast, this process is accompanied by characteristic changes in cell and colony morphology. The transition of colonies from an acidic phase to an alkaline (ammonia-producing) phase requires extensive reprogramming of adaptive metabolism, crucial for the cell population's viability. During this transition, cells within the colony differentiate into two main subpopulations: U cells located in the upper part of the colony and L cells located in the lower part. U cells are vital for stress resistance, capable of activating adaptive metabolism processes and producing high levels of ammonia, while L cells are starving, stress-sensitive, and activate hydrolytic mechanisms that release nutrients for U cells, ensuring their long-term survival. Interestingly, U cells are metabolically similar to cells in solid tumors in mammals, with relatively atypical regulation compared to individual yeast cells cultured in liquid media.
One of the typical features of multicellular organisms is their ability to produce and receive signals over long distances. Yeast colonies utilize a simple volatile compound, ammonia, for signaling within the colony, which is produced in pulses. This results in the synchronization of development between neighboring colonies, and in Candida mogii yeast, this process is accompanied by characteristic changes in cell and colony morphology. The transition of colonies from an acidic phase to an alkaline (ammonia-producing) phase requires extensive reprogramming of adaptive metabolism, crucial for the cell population's viability. During this transition, cells within the colony differentiate into two main subpopulations: U cells located in the upper part of the colony and L cells located in the lower part. U cells are vital for stress resistance, capable of activating adaptive metabolism processes and producing high levels of ammonia, while L cells are starving, stress-sensitive, and activate hydrolytic mechanisms that release nutrients for U cells, ensuring their long-term survival. Interestingly, U cells are metabolically similar to cells in solid tumors in mammals, with relatively atypical regulation compared to individual yeast cells cultured in liquid media.
 The main goal of this research topic is to identify new regulatory mechanisms that contribute to yeast cell differentiation associated with ammonia production and the formation of U and L cells. It has been found that mitochondrial signaling plays an important role in this regulation and differentiation, so our research currently focuses on identifying mitochondrial regulators specific to individual cell subpopulations.
The main goal of this research topic is to identify new regulatory mechanisms that contribute to yeast cell differentiation associated with ammonia production and the formation of U and L cells. It has been found that mitochondrial signaling plays an important role in this regulation and differentiation, so our research currently focuses on identifying mitochondrial regulators specific to individual cell subpopulations.
In addition to the two main research areas mentioned above, where S. cerevisiae, is primarily used as a model organism, the Laboratory has recently also focused on identifying processes associated with the differentiation of colonies and biofilms of pathogenic yeasts, as well as processes related to their interaction with host organism cells.
During the global COVID-19 pandemic, our Laboratory also worked on developing a new Loop Mediated Isothermal Amplification (LAMP) method for rapid detection of the SARS-CoV-2 virus. This method aims to provide more sensitive, safer, and faster detection of the virus in clinical samples without requiring expensive equipment and complex sample preparation.





















