The team of Dr. Veronika Obšilová from the Institute of Physiology of the CAS and Prof. Tomáš Obšil from the Department of Physical and Macromolecular Chemistry, Faculty of Science, Charles University, has described the mechanism of regulation of the E3 ubiquitin ligase Nedd4-2. The study was published in the prestigious journal Nature Communications.
Nedd4-2 is a key enzyme involved in regulating sodium levels by controlling the removal of sodium channels from cell membranes. This function is essential for the regulation of blood pressure and osmotic balance, and its disruption has been linked to diseases such as hypertension, kidney disease and some cancers. A new study by a team from the Institute of Physiology of the CAS (website) and the Faculty of Science, Charles University (website) has for the first time described in detail the structure of the entire Nedd4-2 enzyme and explained the mechanism of its regulation. Using advanced imaging and biochemical methods, the researchers found that the function of Nedd4-2 is blocked by interactions between its domains. The enzyme remains inactive until it binds to the cell membrane in the presence of calcium ions. This leads to the release of inter-domain interactions and the activation of Nedd4-2. The study also showed that the 14-3-3 proteins, which respond to hormonal signals and by their binding block both the enzymatic activity of Nedd4-2 and its ability to bind to the membrane, are also involved in the regulation of Nedd4-2.
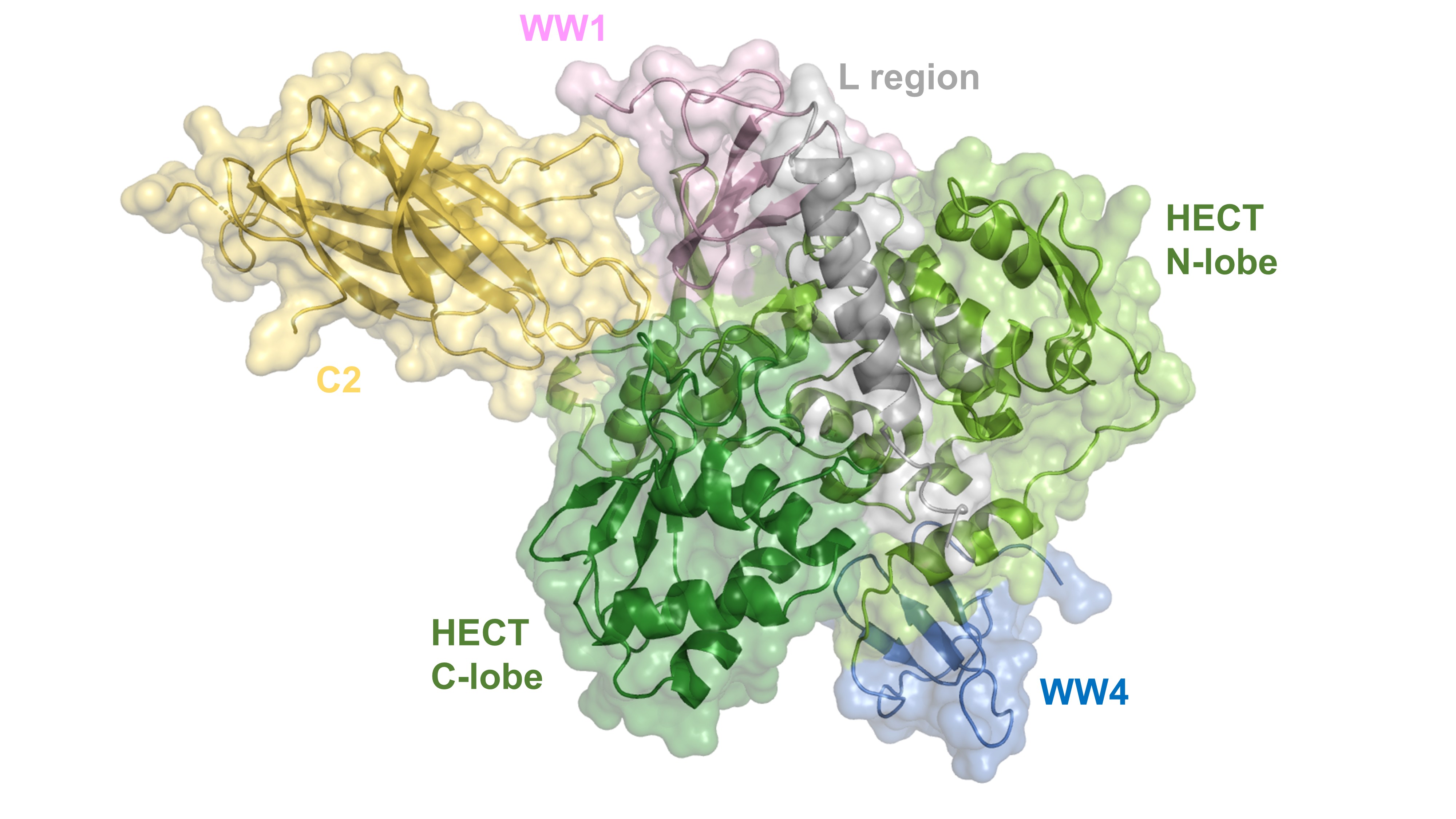
Structure of Nedd4-2 obtained by cryo-EM reconstruction: C2, calcium binding domain (peach); WW1 (pale purple); L region (gray); WW4 (blue); HECT N-lobe, N-lobe of the catalytic HECT domain (light green); HECT C-lobe, C-lobe of the catalytic HECT domain (dark green).
Link: Nat. Comm. 2025, 16, 4875. https://www.nature.com/articles/s41467-025-60207-4





















