Dr. Vojtěch Dočekal and Prof. Jan Veselý, from the Department of Organic Chemistry, have developed a highly effective methodology for the preparation of inherently chiral calix[4]arenes. The study was published in the prestigious journal Nature Communications.
Calix[4]arenes are macrocyclic compounds composed of four benzene rings linked by methylene bridges. These molecules have demonstrated broad utility in materials science, medicinal chemistry, and synthetic applications, including use as selective sensors, drug-delivery agents, and catalysts. However, the synthesis of inherently chiral calix[4]arenes has traditionally relied on the resolution of racemic mixtures, limiting their practical applicability.
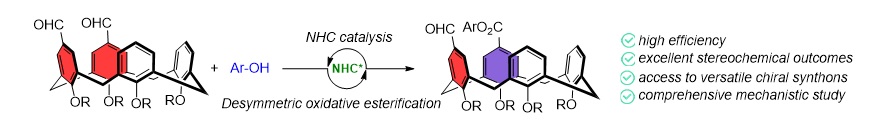
The new methodology, developed by the Group of Asymmetric Synthesis (website), is based on an organocatalytic oxidative esterification of prochiral diformylcalix[4]arenes. This transformation is catalysed by a bifunctional organocatalyst that combines an N-heterocyclic carbene (NHC) with a hydrogen-bond donor, specifically a thiourea. Importantly, this is the first reported example of an efficient catalytic synthesis of inherently chiral calix[4]arenes bearing an ABCC substitution pattern by NHC catalysis.
The study also includes a comprehensive mechanistic investigation, integrating both experimental and computational approaches to elucidate the origin of stereocontrol. Furthermore, the authors demonstrate the synthetic utility of their method through a variety of post-functionalizations of the chiral products, such as the preparation of novel organocatalysts and enantioselective recognition agents.
His research continues the group's ongoing efforts in developing desymmetrization strategies. For instance, a related concept was successfully applied last year to the synthesis of planar chiral [2.2]paracyclophanes, also published in Nature Communications (Nat. Commun. 2024, 15, 3090. https://doi.org/10.1038/s41467-024-47407-0).
Reference: Nat. Commun. 2025, 16, 4443. https://www.nature.com/articles/s41467-025-59781-4.





















