Scientists have uncovered new insights into the protoribosome, a molecular fossil within the ribosome, which plays a key role in the origin of life on Earth. This research, published in Nucleic Acids Research, could reshape our understanding of early ribosomal evolution and its significance for the development of life. One of the study´s lead researchers is Dr. Klára Hlouchová from department of Cell biology of the Faculty of Science, Charles University.
Graphical abstract
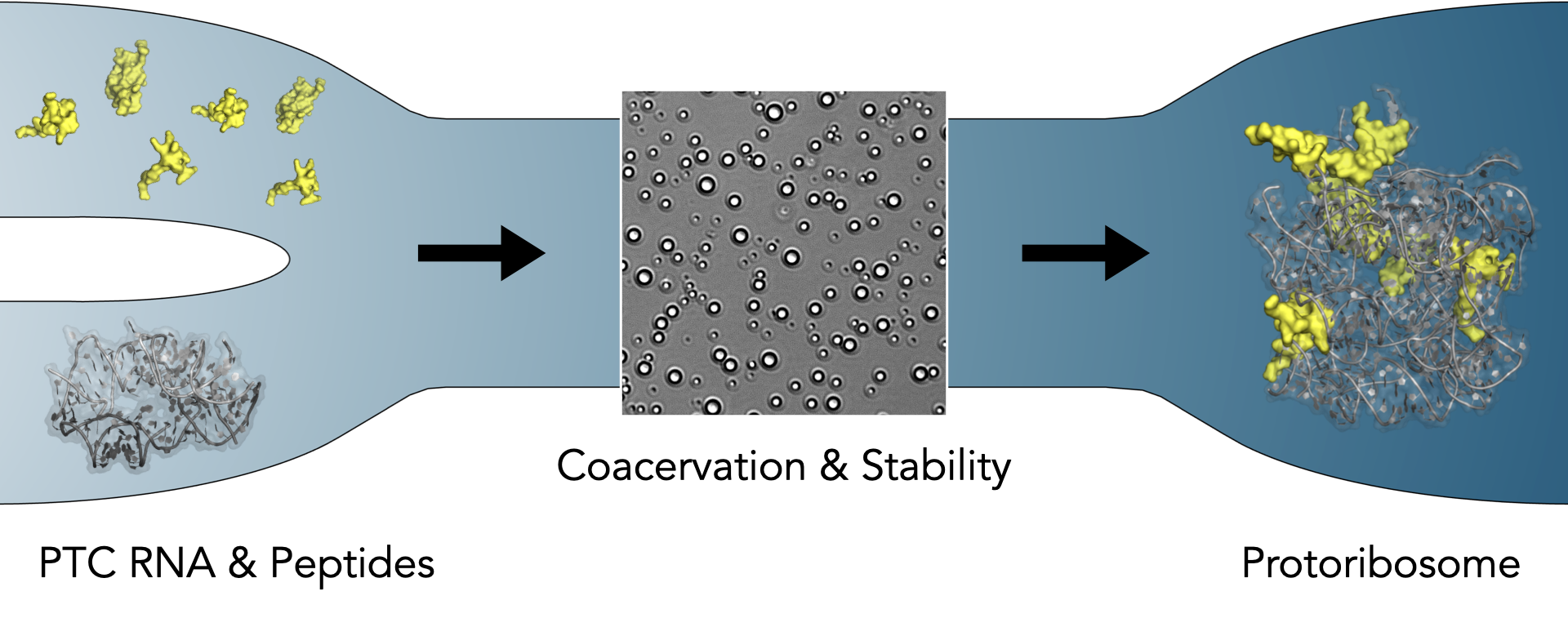
In a groundbreaking study, a cross-disciplinary team of scientists from the Charles University and University of Chemistry and Technology in Prague, University of Milano, and Institute of Science Tokyo (formerly Tokyo Institute of Technology) have demonstrated how peptides (resembling the most ancient fragments of current ribosomal proteins) contribute to the condensation and stability of the protoribosomal RNA.
Background
Life as we know it nowadays relies on the intimate connection among nucleic acids, storing information, proteins, performing countless tasks, and lipids forming surrounding membranes. “These interactions among molecular precursors started to occur more than 4 billion years ago before the first life emerged,” says Dr. Klára Hlouchová from the Charles University, one of the study’s lead researchers. Cells contain ribosomes, molecular machines which produce proteins. Due to their omnipresence and high conservation across all life forms, ribosomes qualify as ancient molecular fossils, attracting evolutionary biologists as the best connection to our biological past.
Key discovery
The protoribosome surrounds the peptidyl transferase center (PTC), which is responsible for peptide bond formation—an essential process in protein synthesis. Previous studies showed that RNA alone could exhibit PTC activity. However, in the ribosomal structure, “tails” of several ribosomal proteins (rPeptides) are located in proximity of the PTC and have been implied as relics of the most ancient peptide species that probably interacted with the protoribosome before the ribosome evolved into the RNA-protein complex as we know it today. The role of these rPeptides has not been studied so far.
This new investigation reveals that the rPeptides have a critical role in driving compartmentalization and hence stability of the protoribosome. Two distinct evolutionary stages of the protoribosomal RNA have been studied here - The 617- and 136-nucleotide (“big” and “small”) constructs. The small construct (linked to the previously shown PTC activity) is more structurally flexible and while it interacts with rPeptides with lower specificity, the peptides induce coacervation—a process that helps form liquid-like droplets—across a wide concentration range. This, in turn, protects RNA from degradation. The big construct, on the other hand, is structurally more defined, as shown by atomistic computer simulations performed by the group of associate professor Michal H. Kolář at University of Chemistry and Technology in Prague. The protoribosomal RNA in the big construct interacts with the rPeptides with higher specificity and coacervation of this complex is less prominent than of the small construct.
The distinct properties of the two protoribosomal stages suggest that rPeptides initially provided compartmentalization and prevented RNA degradation, preceding the emergence of specific RNA-protein interactions crucial for the structural integrity of the contemporary ribosome.
Evolutionary Implications
The research suggests that the interaction between RNA and proteins before the first life emerged, offered a significant biophysical advantage, especially by providing compartmentalization and preventing RNA from degradation. These early RNA-protein interactions are seen as a precursor to the more complex RNA-protein relationships that are essential for ribosomal structural integrity today.
 Dr. Klára Hlouchová, one of the study’s lead researchers, explains, “Our findings imply that peptides play a vital role in driving condensation and stabilizing the protoribosome. This sheds light on how fundamental life processes may have been protected and compartmentalized in a prebiotic world.” “At the same time, the spontaneous formation of concentrated droplets depends in a subtle way on the RNA sequence and structure, implying that it is rather specific for the ribosomal particles” adds Prof. Giuliano Zanchetta, a co-lead researcher of the study.
Dr. Klára Hlouchová, one of the study’s lead researchers, explains, “Our findings imply that peptides play a vital role in driving condensation and stabilizing the protoribosome. This sheds light on how fundamental life processes may have been protected and compartmentalized in a prebiotic world.” “At the same time, the spontaneous formation of concentrated droplets depends in a subtle way on the RNA sequence and structure, implying that it is rather specific for the ribosomal particles” adds Prof. Giuliano Zanchetta, a co-lead researcher of the study.
END
This press release is based on the study, "The interplay between peptides and RNA is critical for protoribosome compartmentalization and stability," published in Nucleic Acids Research.
The research was funded by Human Frontiers Science Foundation and the 4EU+ European University Alliance.
_________________________________________________________________________________
Simone Codispoti, Tomoko Yamaguchi, Mikhail Makarov, Valerio G Giacobelli, Martin Mašek, Michal H Kolář, Alma Carolina Sanchez Rocha, Kosuke Fujishima, Giuliano Zanchetta, Klára Hlouchová, The interplay between peptides and RNA is critical for protoribosome compartmentalization and stability, Nucleic Acids Research, 2024;, gkae823, https://doi.org/10.1093/nar/gkae823





















