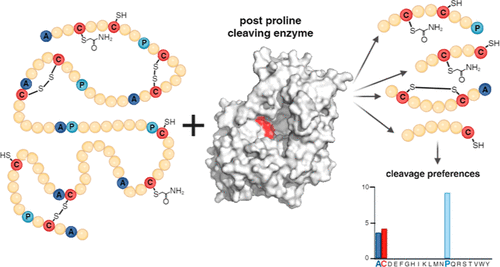
Proteolysis is an essential step for sample processing in mass spectrometric analysis of proteins, their modifications and structural aspects (so-called structural proteomics). A team from the BioCeV Centre, including members of our Department Zuzana Kalaninová, Jasmína Mária Portašiková, Barbora Jirečková, Marek Polák a Petr Novák, has recently discovered that an important group of enzymes, considered specific for cleavage after proline and alanine (Post Proline Cleaving Enzymes - PPCEs), also have selectivity for reduced cysteine. These are proteases from black mold Aspergillus niger. The cleavage after proline makes these enzymes a promising proteolytic tool, since proline is a forbidden amino acid for other enzymes, and must not be present at the cleavage site. The selectivity towards reduced cysteine opens a whole new field of application for PPCEs - e.g. for the analysis of disulfide bridges or cysteine modification. However, the impact of this discovery does not end there. PPCEs play an essential role in regulating biological processes (dipeptidyl peptidase, cathepsin) or treating gluten intolerance and celiac disease.
Full article: https://pubs.acs.org/doi/10.1021/acs.analchem.4c04277





















