Publikace podle roku
- Publikace článku skupiny Jana Veselého v Journal of Organic Chemistry:
Abstrakt: Stereoselective synthesis of spirocyclic compounds containing heterocyclic motifs represents a formidable challenge in enantioselective synthesis. Here, we present a cascade reaction between α,β-unsaturated aldehydes and isoxazolones under synergistic catalysis of a chiral secondary amine and a palladium(0) catalyst. This strategy allows access to chiral spiroisoxazolone derivatives with a large substrate scope tolerance and high levels of diastereoselectivity (dr up to 20:1) and enantioselectivity (up to 99% ee). Furthermore, the utility of this methodology is showcased by the transformation of chiral spiroisoxazolones into structurally attractive and enantiomerically enriched cyclopentene carboxylic acids with two stereogenic centers.
- Publikace článku skupiny Martina Kotory v magazinu Chemistry Views a časopise Chemistry Europe:
Abstrakt: A general protocol for the photorearrangement of substituted benzo- and naphthoquinones using monochromatic blue light irradiation in solution was developed. During this process, the quinone ring is transformed into the hydroquinone, while the substituent undergoes desaturation or annulation onto the proximal oxygen of the formed hydroquinone. We found that desaturation was preferred for acyclic saturated substituents, such as alkyl groups, and the tendency for annulation was observed for both cyclic and acyclic unsaturated substituents (allyl, alkenyl, aryl). The diversity of the participating substituents and the functional group tolerance of this transformation allowed us to prepare 10 natural phenolic compounds, suggesting that visible light may likewise induce their formation from the respective quinones in natural sources.

- Publikace článku skupiny Radima Hrdiny v Organic and Biomolecular Chemistry:
C–H amination of enolizable and nonenolizable ketones
Abstrakt: We present a method for the amination of enolizable and non-enolizable ketones in the alpha (or beta) position to the carbonyl group. This approach is based on the conversion of the corresponding cyanohydrins to carbonazidates, precursors for thermal intramolecular nitrene insertion reactions into the adjacent C–H bond. Hydrolysis of the resulting carbamates under basic conditions with simultaneous regeneration of the carbonyl group yields amino ketones.

- Publikace článku skupiny Martina Kotory v Chemical Communications:
Abstrakt: A series of helical quinolizinium salts were prepared utilizing Rh-catalyzed [2+2+2]cyclotrimerization and C–H activation processes as the crucial synthetic steps. The cyclotrimerization of appropriately substituted diynes with trimethylsilylethyne under Rh-catalyzed conditions provided the 1-arylisoquinolines in up to 61% isolated yields. Their Rh-catalyzed C–H activation/annulation with various aryl and alkyl disubstituted alkynes gave rise to [7]-helical quinolizinium salts in high isolated yields (up to 93%). Enantioselective C–H activation was also tried with asymmetric induction up to 62% ee. The respective boron and platinum complexes of 1-arylisoquinolines were prepared as well. All prepared compounds exhibit fluorescence in the orange-red light region (606–682 nm) with ΦFs 28–99%.

- Publikace článku skupiny Jana Veselého v Advanced Synthesis and Catalysis:
Abstrakt: In this work, we present an organocatalytic spiroannulation method that provides access to spirocyclic benzofuran-2-ones. The reaction proceeds via the generation of an azolium dienolate from an α-bromo-α,β-unsaturated aldehyde using a chiral N-heterocyclic carbene, followed by annulation with benzofuran-2-one-derived alkenes. The resulting chiral spirobenzofuranone-fused cyclohexenones were obtained in 77–99% ee and dr from 1.2:1 to 20:1. The utility of the protocol was underscored by gram-scale reaction and follow-up transformations.

- Publikace článku skupiny Jana Veselého v Journal of Organic Chemistry:
Abstrakt: Azlactone is an important starting material for synthesizing amino acids containing a quaternary α-carbon. In this study, we have developed a sequential “one-pot” procedure involving an enantioselective spirocyclization reaction followed by acidic azlactone opening, which led to amino acid derivatives. The key step of this procedure is a spirocyclization between propargylated azlactones and enals by using a cooperative catalytic approach that combines chiral secondary amine and achiral Pd(0) complexes. The final acid opening of the azlactone motif allows isolation of the corresponding amino acid derivatives as major diastereoisomers in yields ranging from 37% to 70% with enantioselectivities of 85–97% ee. These synthesized amino acid derivatives hold great potential in the pharmaceutical and bioactive compound industries. Moreover, the final amino acid products with a cyclopentene moiety can be further derivatized, opening up even more possibilities for their application.

- Publikace článku skupiny Lukáše Rýčka v Chem Plus Chem:
Abstrakt: We report a synthesis of silver complexes bearing chelating bidentate N-heterocyclic carbene, with various substitutions at the terminal positions of the imidazole moiety of the NHC units. The long aliphatic substituents proved to be beneficial in terms of the synthetic efficiency of the complexes, compared to previously reported methyl substitution. The complexes demonstrated excellent suitability for the KA2 coupling reaction, providing quaternary carbon-containing propargylic amines in yields up to 95 %, under solvent-free conditions. The method showed high tolerance for a wide range of substrates, including naturally occurring ketones, underscoring its practicality. To our knowledge, this represents the first use of a well-defined silver species in KA2 coupling, marking an advancement in the field.
- Publikace článku skupiny Lukáše Rýčka v ACS Bio & Med Chem Au:
Abstrakt: The development of multitargeted drugs represents an innovative approach to cancer treatment, aiming to enhance drug effectiveness while minimizing side effects. Herein, we sought to elucidate the inhibitory effect of selagibenzophenone B derivatives on the survival of cancer cells and dual topoisomerase I/II enzyme activity. Results demonstrated that among the compounds, SelB-1 selectively inhibited the proliferation and migration of prostate cancer cells while exhibiting minimal effects on healthy cells. Furthermore, SelB-1 showed a dual inhibitory effect on topoisomerases. Computational analyses mirrored the results from enzyme inhibition assays, demonstrating the compound’s strong binding affinity to the catalytic sites of the topoisomerases. To our surprise, SelB-1 did not induce apoptosis in prostate cancer cells; instead, it induced autophagic gene expression and lipid peroxidation while reducing GSH levels, which might be associated with ferroptotic death mechanisms. To summarize, the findings suggest that SelB-1 possesses the potential to serve as a dual topoisomerase inhibitor and can be further developed as a promising candidate for prostate cancer treatment.
- Publikace článku skupiny Lukáše Rýčka v Chem Plus Chem:
Abstrakt: This work reports a biomimetic synthesis of polyarylated fluorene derivatives. The molecules are formed via intramolecular electrophilic aromatic substitution, resembling a cyclization leading towards the natural selaginpulvilins from selaginellins. The scope of the reaction was investigated, and the products were obtained in 60–95 % yields. Some of the compounds decompose to a stable radical. We investigated the nature and the origin of the radical using experimental methods, including EPR or electrochemical measurements, as well as theoretical methods, such as DFT calculations. Based on our observations, we hypothesize, that phenoxy radicals are formed in the first instance, which however undergo internal rearrangement to thermodynamically more stable carbon-centered radicals. The preliminary data also show the cytotoxic properties of some of the molecules.

- Publikace článku skupiny Lukáše Rýčka v New Journal of Chemistry:
Abstrakt: We report the synthesis of a heterogenous silver catalyst stabilized by bidentate N-heterocyclic carbene ligands. The heterogeneous nature of the catalyst is inherently derived from the unprecedented internal structural features observed for the catalyst. X-ray studies show that the silver complex forms a two-dimensional cross-linked system, where one dimension of the network is formed by inorganic polymer strings and the second direction is formed by the interconnection of these inorganic strings with NHC bidentate ligands. This organized internal arrangement renders the complex insoluble in many common organic solvents and enables its utilization as a reusable heterogeneous catalyst in multicomponent A3 coupling reactions. Notably, propargylic amines were obtained with yields of up to 97%, and the catalyst demonstrated reusability for six cycles. These results can facilitate the further development of heterogeneous NHC catalysts.

- Publikace článku skupiny Jiřího Míška v Journal of Organic Chemistry:
Abstrakt: This study unveils a new catalytic crossover reaction of sulfinamides. Leveraging mild acid catalysis, the reaction demonstrates a high tolerance to structural variations, yielding equimolar products across diverse sulfinamide substrates. Notably, small sulfinamide libraries can be selectively oxidized to sulfonamides, providing a new platform for ligand optimization and discovery in medicinal chemistry. This crossover chemotype provides a new tool for high-throughput experimentation in discovery chemistry.
- Knižní publikace Martina Kotory v nakladatelství Springer:
Abstrakt: Tato kniha obsahuje 8 kapitol shrnující výskyt, syntézu a využití sloučenin obsahujících cyklopentadienovou funkční skupinu za uplynulá desetiletí. Kniha je určena nejen široké odborné veřejnosti z řad chemiků zabývajících se přírodními látkami, organickou syntézou, komplexy přechodných kovů a katalytickými procesy, ale i studentům, kteří zde mohou nalézt a načerpat řadu cenných a užitečných informací. Autory jednotlivých kapitol jsou odborníci, včetně přispěvatelů z naší fakulty, jenž se aktivně zabývají uvedenou tématikou.

Abstrakt: Innovative approaches to controlled nucleobase-modified RNA synthesis are urgently needed to support RNA biology exploration and to synthesize potential RNA therapeutics. Here we present a strategy for enzymatic construction of nucleobase-modified RNA based on primer-dependent engineered thermophilic DNA polymerases – SFM4-3 and TGK. We demonstrate introduction of one or several different base-modified nucleotides in one strand including hypermodified RNA containing all four modified nucleotides bearing four different substituents, as well as strategy for primer segment removal. We also show facile site-specific or segmented introduction of fluorophores or other functional groups at defined positions in variety of RNA molecules, including structured or long mRNA. Intriguing translation efficacy of single-site modified mRNAs underscores the necessity to study isolated modifications placed at designer positions to disentangle their biological effects and enable development of improved mRNA therapeutics. Our toolbox paves the way for more precise dissecting RNA structures and functions, as well as for construction of diverse types of base-functionalized RNA for therapeutic applications and diagnostics.

- Publikace článku skupiny Martina Kotory v Asian Journal of Organic Chemistry:
Abstrakt: Attempts to carry out intramolecular annulation of 9,10-diarylbenzo[h]quinolines and 9,10-di(hetero)arylphenanthrenes to aromatics with expanded π-conjugated systems by using different methods are described. The starting compounds (9,10-diarylbenzo[h]quinolines and 9,10-di(hetero)arylphenanthrenes) were prepared by C−C bond activation in 1-azabiphenylene or biphenylene followed by insertion of internal alkynes. Interestingly, unlike in purely carbon-based aromatics, the course of the annulation turned out to be highly dependent on the structure of maternal compounds. In a handful of cases were obtained the expected or desired products. In others, unexpected rearrangements of the basic molecular frameworks were observed.

- Publikace článku skupiny Jana Veselého v Nature Communications:
Abstrakt: Planar chiral [2.2]paracyclophanes consist of two functionalized benzene rings connected by two ethylene bridges. These organic compounds have a wide range of applications in asymmetric synthesis, as both ligands and catalysts, and in materials science, as polymers, energy materials and dyes. However, these molecules can only be accessed by enantiomer separation via (a) time-consuming chiral separations and (b) kinetic resolution approaches, often with a limited substrate scope, yielding both enantiomers. Here, we report a simple, efficient, metal-free protocol for organocatalytic desymmetrization of prochiral diformyl[2.2]paracyclophanes. Our detailed experimental mechanistic study highlights differences in the origin of enantiocontrol of pseudo-para and pseudo-gem diformyl derivatives in NHC catalyzed desymmetrizations based on whether a key Breslow intermediate is irreversibly or reversibly formed in this process. This gram-scale reaction enables a wide range of follow-up derivatizations of carbonyl groups, producing various enantiomerically pure planar chiral [2.2]paracyclophane derivatives, thereby underscoring the potential of this method.
- Publikace článku ve spolupráci dvou skupin Radima Hrdiny a Jindřicha Jindřicha v Beilstein Journal of Organic Chemistry:
Abstrakt: 13C NMR spectroscopic analyses of Cs symmetric guest molecules in the cyclodextrin host cavity, combined with molecular modelling and solid-state X-ray analysis, provides a detailed description of the spatial arrangement of cyclodextrin host–guest complexes in solution. The chiral cavity of the cyclodextrin molecule creates an anisotropic environment for the guest molecule resulting in a splitting of its prochiral carbon signals in 13C NMR spectra. This signal split can be correlated to the distance of the guest atoms from the wall of the host cavity and to the spatial separation of binding sites preferred by pairs of prochiral carbon atoms. These measurements complement traditional solid-state analyses, which rely on the crystallization of host–guest complexes and their crystallographic analysis.
- Publikace článku skupiny Lukáše Rýčka v Applied Organometallic Chemistry:
Abstrakt: We present a synthesis of novel silver complexes stabilized by bidentate ligands based on N-heterocyclic carbenes (NHC) linked with a bisamide linker. The ligand stabilizes the silver ion in a rare chelating mode. The synthesis of the complex depends on the equimolar ratio of the silver source and the ligand precursor. In case the excess of the silver source is used, the reaction leads to the formation of an unprecedented tetranuclear silver complex, stabilized by two equivalents of the ligand, where each of the silver atoms is coordinated by one NHC and one amide moiety. The silver complexes were applied as a catalyst in a multicomponent A3coupling and proved to be a very efficient catalyst. The reaction provided desired products in yields up to 96%, and the use of low catalytic loading, as low as 0.1 mol%, was possible without significantly compromising the effectivity of the reaction. Moreover, the complexes showed broad spectra of antimicrobial activity, with minimal inhibitory concentrations in the range of 1 to 31 μg/ml against several Gram-positive and Gram-negative bacteria and fungi. Presented complexes represent synthetically challenging molecules, which show great applicability in catalysis and outstanding potential as antimicrobial agents.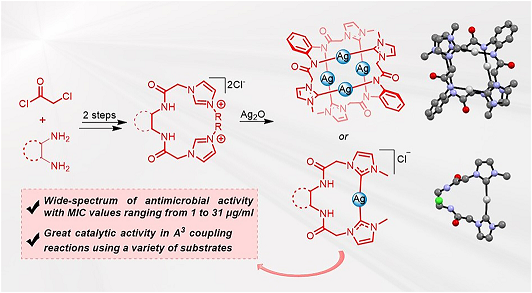
- Publikace článku skupiny Elišky Matoušové v European Journal of Organic Chemistry:
Abstrakt: This study presents the synthesis of analogues of bioactive Amaryllidaceae alkaloids that contain a five-membered ring C and a quaternary carbon centre. The synthesis was carried out in a divergent manner, starting from a common intermediate, keto aldehyde. This intermediate was obtained by oxidative cleavage of a carbocyclic ring present in the previously synthesised tetracyclic compounds. Subsequent closure of a new heterocyclic ring B using different methods led to products with structural cores found in naturally occurring alkaloids such as pretazettine, tazettine or macronine. In addition, an analogue of egonine was prepared from the same intermediate. Our approach thus provides access to multiple structural types of potentially bioactive molecules.
- Publikace článku skupiny Ondřeje Baszczyňského v Green Chemistry:
Abstrakt: Organophosphorus compounds containing hydrolytically and metabolically stable C(sp3)– and C(sp2)–P bonds are widely used as reagents, ligands, pesticides, herbicides, flame retardants, surface modifiers, and antiviral and anticancer drugs. These applications rely on efficient C(sp3)– and C(sp2)–P bond-forming reactions. However, currently available C(sp2)–P cross-coupling protocols require high catalyst loadings and temperatures, as well as environmentally unsustainable and harmful organic solvents (e.g., N,N-dimethylformamide, DMF). Herein, we disclose a conceptually novel strategy for performing multimetallic Pd/Ni- and dual-ligand Pd-catalyzed C(sp2)–P cross-coupling reactions in aqueous micelles under mild and environmentally friendly conditions. Micellar catalysis in water enables C(sp2)–P cross-coupling while avoiding environmentally unsustainable organic solvents, thereby reducing organic waste generation. Such micellar C(sp2)–P cross-coupling reactions tolerate various functional groups and provide access to structurally diverse (hetero)aryl (thio)phosphonates, phosphinates and phosphine oxides using inexpensive commercial materials and catalysts. Moreover, C(sp2)–P cross-coupling reactions of medically relevant substrates and drugs under late-stage functionalization settings and multistep one-pot processes highlight the potential applications of this experimental paradigm.

- Publikace review skupiny Radima Hrdiny v Molecules:
Abstrakt: This review summarizes achievements in the synthesis of 1,2-disubstituted adamantane derivatives by the construction of the tricyclic framework either by total synthesis or by ring expansion/contraction reactions of corresponding adamantane homologues. It is intended to complement reviews focusing on the preparation of 1,2-disubstituted derivatives by C–H functionalization methods.
- Publikace článku skupiny Lukáše Rýčka v ChemistrySelect:
Abstrakt: We report a modular synthetic approach towards novel derivatives of the naturally occurring arylated benzophenone selagibenzophenone A. The initial strategy for the construction of the carbon framework of the derivatives relied on the Suzuki reaction of 2,4,6-tribromobenzonitrile, and the addition of the aryl lithium species to nitrile to generate imine. However, the formed imines showed remarkable stability toward hydrolysis. Therefore, Suzuki cross-coupling was carried out with 2,4,6-tribromobenzaldehyde and the subsequent addition of organometallic species to the aldehyde. Oxidation of the resulting alcohol ensured the access to desired ketones. The importance of the developed modular strategy is underlined by the discovery of several derivatives with selective cytotoxic effects and potential anti-inflammatory activity superior to the effect of the natural product.
- Publikace review skupiny Jana Veselého v Chemical Record:
Abstrakt: Over the last ten years, the combination of organocatalysis with transition metal (TM) catalysis has become one of the most important toolboxes used for synthesizing optically pure compounds containing chiral quaternary centers, including spiro heterocyclic molecules. The dominant method in the enantioselective synthesis of spiro heterocyclic compounds based on synergistic catalysis includes chiral aminocatalysis and NHC catalysis, as already established covalent organocatalytic strategies. Another area of organocatalysis widely combined with TM catalysis producing enantiomerically enriched spiro heterocyclic compounds is non-covalent catalysis, dominated by chiral phosphoric acids, thiourea, and squaramide derivatives. This review article aims to summarize enantioselective methods used for constructing spirocyclic heterocycles based on a combination of organocatalysis and transition metal catalysis.

- Císařová, I.; Holovko-Kamoshenkova, O. M.; Hrdina, R.; Koucký, F.; Machalický, O. Annulated carbamates are precursors for the ring contraction of the adamantane framework. RSC Advances [online] 2022, 12 (48), 31056-31060.
- Konvalinka, J.; Kožíšek, M.; Kráľ, M.; Machara, A.; Majer, P.; Reiberger, R. Bioisosteres of Luteolin: New Class of Potential Inhibitors of Influenza Virus RNA-Polymerase, 2022.
- Bingel, S.; Caivano, I.; Císařová, I.; Kotora, M.; Nečas, D. Catalytic approach to unsymmetrical [7]-helical indenofluorenes: Cyclotrimerization vs. dehydro-Diels-Alder reaction pathways. Catalysis Today 2022, 390-391 (May), 48-56.
- Bhosale, V. Anandrao; Císařová, I.; Kamlar, M.; Veselý, J. Catalytic asymmetric addition to cyclic N-acyl-iminium: Access to sulfone-bearing contiguous quaternary stereocenters. Chemical Communications 2022, 58 (71), 9942-9945.
- Čikoš, A.; Degač, M.; Gredičak, M.; Topolovčan, N. Chemoselective and Regioselective Synthesis of Spiroisoindolinone Indenes via an Intercepted Meyer–Schuster Rearrangement/Intramolecular Friedel–Crafts Alkylation Relay. Journal of Organic Chemistry 2022, 87 (5), 3712-3717.
- Asanuma, Y.; Císařová, I.; Kotora, M.; Manca, G.; Moss, R.; Ulč, J. Computational, Mechanistic, and Experimental Insights into Regioselective Catalytic C–C Bond Activation in Linear 1-Aza-[3]triphenylene. ACS Omega [online] 2022, 7 (10), 8665-8674.
- Dian, J.; Fourmentin, S.; Jindřich, J.; Palágyi, A. Cyclodextrin-based Schiff base pro-fragrances: Synthesis and release studies. Beilstein Journal of Organic Chemistry 2022, 18 (September), 1346-1354.
- Císařová, I.; Jacko, J.; Kotora, M.; Rulíšek, L.; Staś, M. Ir-Catalyzed Cycloaddition of Tribenzocyclyne with Biphenylenes. Journal of Organic Chemistry 2022, 87 (1), 744-750.
- Jindřich, J.; Kasal, P. Kinetics of Nucleophilic Substitution of Compounds Containing Multiple Leaving Groups Bound to a Neopentyl Skeleton. ACS Omega [online] 2022, 7 (23), 20137-20144.
- Ausserlechner, M. J.; Dočekal, V.; Hagenbuchner, J.; Horváth, M.; Kaiser, N.; Kohoutová, K.; Mandal, R.; Obšil, T.; Obšilová, V.; Tekel, A.; et al. Lengthening the Guanidine–Aryl Linker of Phenylpyrimidinylguanidines Increases Their Potency as Inhibitors of FOXO3-Induced Gene Transcription. ACS Omega [online] 2022, 7 (38), 34632-34646.
- Ausserlechner, M.; Dočekal, V.; Kohoutová, K.; Obšil, T.; Obšilová, V.; Tekel, A.; Veselý, J. Modulating FOXO3 transcriptional activity by low-molecular compounds. FEBS Open Bio [online], 2022, 12, 233-233.
- Císařová, I.; David, T.; Dračínský, M.; Jirák, D.; Jurok, R.; Kretschmer, J.; Kuchař, M.; Polášek, M.; Socha, O.; Vít, M. Paramagnetic encoding of molecules. Nature Communications [online] 2022, 13 (1), nestránkováno.
- Baszczyňski, O.; Černý, J.; Čmoková, A.; Grobárová, V.; Kolařík, M.; Procházková, E.; Slapničková, M.; Štěpánek, O.; Zíková, A. Piperazine-Modified Ketoconazole Derivatives Show Increased Activity against Fungal and Trypanosomatid Pathogens. ChemMedChem 2022, 17 (21), nestránkováno.
- Hocek, M.; Jauset-Rubio, M.; Kodr, D.; Ortiz, M.; O'Sullivan, C. Kathleen; Simonova, A. Solid-phase recombinase polymerase amplification using ferrocene-labelled dNTPs for electrochemical detection of single nucleotide polymorphisms. Biosensors and Bioelectronics 2022, 198 (February), nestránkováno.
- Císařová, I.; Dočekal, V.; Gyepes, R.; Hrabovský, J.; Koberová, T.; Rios, R.; Veselý, J.; Vopálenská, A. Stereoselective cyclopropanation of boron dipyrromethene (BODIPY) derivatives by an organocascade reaction. Advanced Synthesis and Catalysis 2022, 364 (5), 930-937.
- Bouřa, E.; Dejmek, M.; Chalupská, D.; Klíma, M.; Matoušová, M.; Mertlíková-Kaiserová, H.; Misehe, M.; Nencka, R.; Šála, M. Structure-based design and modular synthesis of novel PI4K class II inhibitors bearing a 4-aminoquinazoline scaffold. Bioorganic and Medicinal Chemistry Letters 2022, 76 (November), nestránkováno.
- Mateus, M. Alexandre; Kunák, D.; Rýček, L. Synthesis and Structure Confirmation of Selagibenzophenone C. European Journal of Organic Chemistry 2022, 2022 (11), nestránkováno.
- Hocek, M.; Ondruš, M.; Sýkorová, V. Traceless enzymatic synthesis of monodispersed hypermodified oligodeoxyribonucleotide polymers from RNA templates. Chemical Communications 2022, 58 (80), 11248-11251.